Acetanilide Solubility in Water
In distilled water nearly 1 log of the virus was inactivated in 4 min at a 4-min ozone residual of approximately 023 mgliter. The chemistry involved in the titrimetric exercises.

Solubility Of A Acetanilide B Phenacetin And C Paracetamol In Download Scientific Diagram
Reflux the mixture for 20-30 min.
. O H HCN. The double bond from the aldehyde attacks the water and adds another OH to the aldehyde. Synthesis of anthranilic acid 49 25.
Acetanilide is an odourless solid chemical of leaf or flake-like appearance. Biological hydrolysis is the cleavage of biomolecules where a water molecule is. Synthesis of barbituric acid 52 28.
The term is used broadly for substitution elimination and solvation reactions in which water is the nucleophile. Use the References to access important values if needed The hydroxide ion concentration in an A. Cations Pb 2 Cu 2 AI 3 Fe 3 Zn 2 Ni 2 Ca 2 Ba 2.
Given the hydroxide ion concentration OH-aq 0054 M. Pharmacist Only Medicine Substances the safe use of which requires. Synthesis of oxalic acid 43 19.
Place 30 g of p-nitroacetanilide and 150 ml of 70 H 2 SO 4 prepared by adding 100 ml conc. In river water the inactivation was in excess of 9999 in 4 min when the ozone concentration also measured at 4 min exceeded 03 mgliter. Aspirin is also used long-term to help prevent further heart attacks ischaemic strokes and blood clots in people at high risk.
Hydrolysis h aɪ ˈ d r ɒ l ɪ s ɪ s. PH scale common ion effect hydrolysis of salts and pH of their solutions the solubility of sparingly soluble salts and solubility products buffer solutions. What would be the maximum theoretical percent recovery from crystallization of 500 g of solid X from.
Ozone and its reactions. Cyanuric acid is formed by drawing a water molecule from triiot It is false. The chemistry involved in the preparation of the following.
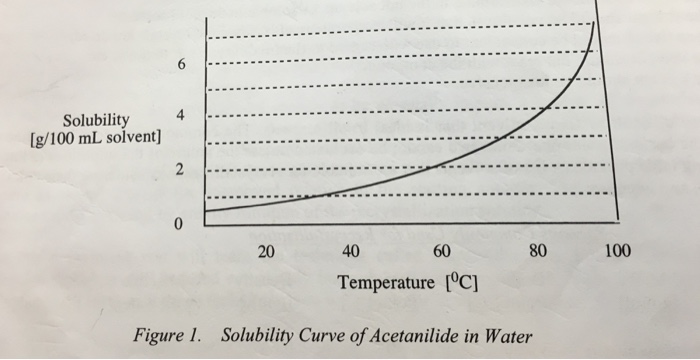
The solubility of acetanilide in hot water 55 g100 ml at 100 o C is not very great and its solubility in cold water 053 g 100 ml at 0 o C is significant. Acetanilide pnitroacetanilide aniline yellow iodoform. Acids bases and the use of indicators oxalic-acid vs KMnO4 Mohrs salt vs KMnO4.
Green house effect and global warming. Click to see the answer. Depletion of ozone layer and its effects.
Synthesis of picric acid 50 CONTENTS. Enter the email address you signed up with and well email you a reset link. 121-61-9 - PKOFBDHYTMYVGJ-UHFFFAOYSA-N - N4-Acetylsulfanilamide - Similar structures search synonyms formulas resource links and other chemical information.
The ozone was measured by the iodide titration without stripping of the. Electrons from one of the OH groups attack the chromium forming a complex with the chromic acid and the. Acid to 75 ml water carefully in a round-bottomed flask.
So the correct Q. O-Nitroacetanilide remains in the filtrate due to its high solubility in water. Aspirin also known as acetylsalicylic acid ASA is a medication used to reduce pain fever andor inflammation.
Acetanilide p-nitro acetanilide aniline yellow iodoform. Pharmacy Medicine Substances the safe use of which may require advice from a pharmacist and which should be available from a pharmacy or where a pharmacy service is not available from a licensed person. Bronsted - Lowry and Lewis and their ionization acid-base equilibria including multistage ionization and ionization constants ionization of water.
And pour the hot solution into 1000 ml of. Assuming the solution is chilled at 0 o C. It is also known as N -phenylacetamide acetanil or acetanilid and was formerly known by the trade name Antifebrin.
Synthesis of phenytoin 45 21. Synthesis of hippuric acid 51 27. Redox Reactions and.
Synthesis of pbromo acetanilide 42 18. What would be the maximum theoretical percent recovery from the crystallization of 50 g of acetanilide from 100 ml water. Specific inflammatory conditions which aspirin is used to treat include Kawasaki disease pericarditis and rheumatic fever.
Mohrs salt potash alum and Organic compounds. Pahlavan 3 Example 1- The solubility of solid X in hot water 550 g100 ml at 100 o C is not very great and its solubility in cold water 053 g100ml at 0 o C is significant. Synthesis of paracetamol 47 23.
Synthesis of aniline 44 20. Green Chemistry Chemicals in medicine health-care and food. CHEM 2423 Recrystallization of Benzoic Acid Dr.
Synthesis of benzoic acid 48 24. Soil water and air pollution. Chemical principles involved in the qualitative salt analysis.
Synthesis of hydantoin 46 22. Preparation of pNitroaniline from p-Nitroacetanilide. What is the major product of this reaction.
Chemistry involved in the titrimetric excercises Acids bases and the use of indicators oxalic-acid vs KMnO 4 Mohrs salt vs KMnO 4. JEE Mains Chapter wise Practice Questions Last 30 Years with 5000 Questions for online practice. This Schedule is intentionally blank.
From Ancient Greek hydro- water and lysis to unbind is any chemical reaction in which a molecule of water breaks one or more chemical bonds. Chemical reactions in atmosphere.

Predicting The Excess Solubility Of Acetanilide Acetaminophen Phenacetin Benzocaine And Caffeine In Binary Water Ethanol Mixtures Via Molecular Simulation The Journal Of Chemical Physics Vol 142 No 4

Solved Based On The Graph How Much Boiling Water Is Needed Chegg Com
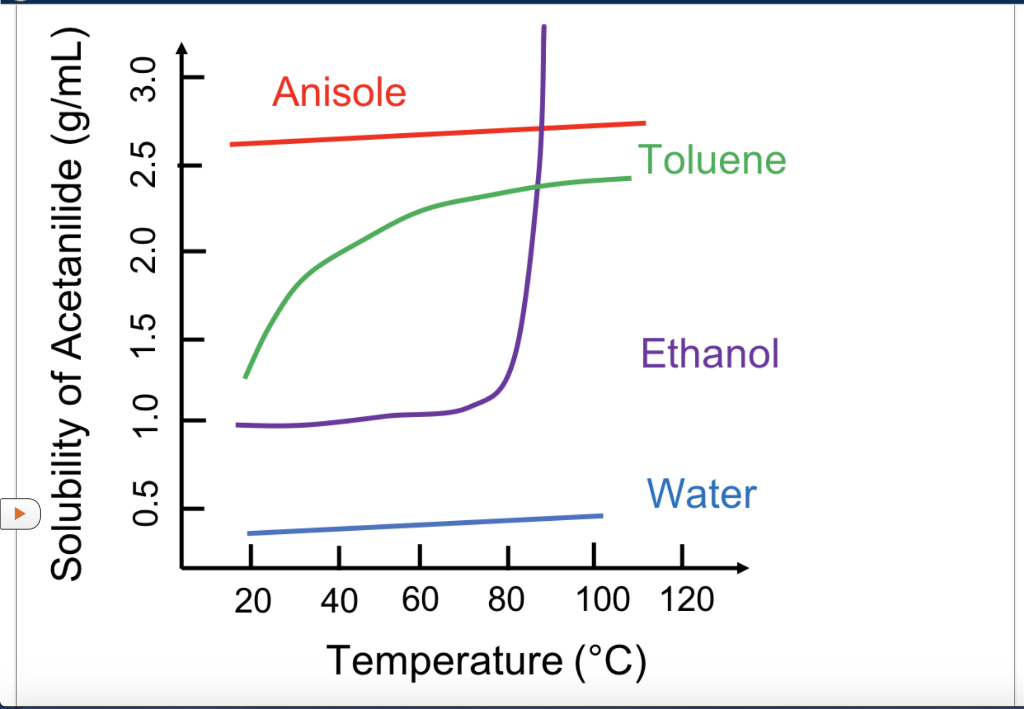
Solved You Can See A Graph Showing Solubility Vs Chegg Com

Predicting The Excess Solubility Of Acetanilide Acetaminophen Phenacetin Benzocaine And Caffeine In Binary Water Ethanol Mixtures Via Molecular Simulation The Journal Of Chemical Physics Vol 142 No 4
No comments for "Acetanilide Solubility in Water"
Post a Comment